Describe the Emission Spectrum of Hydrogen Ib Chemistry
Which statements are correct for the emission spectrum of the Hydrogen atom. Outline how this spectrum is related to the energy levels in the hydrogen atom.
Ib Sl And Hl Chemistry 2 3 3 Explain How The Lines In The Emission Spectrum Of Hydrogen Are Related To Electron Energy Levels
II and III only D.

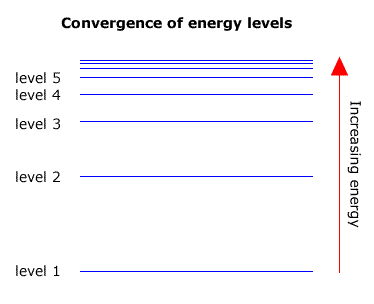
. I II and III. It is possible to detect patterns of lines in both the ultra-violet and infra-red regions of the spectrum as well. The lines converge at lower energies.
Lines are produced when electrons move from higher to lower energy levels. I and II only. In discharge tube under low pressure 10⁴- atm Then it emits light which is analysed by Spectroscope.
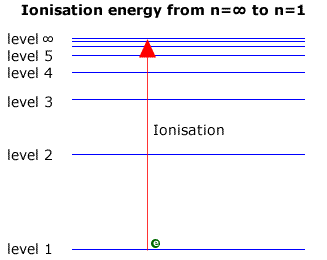
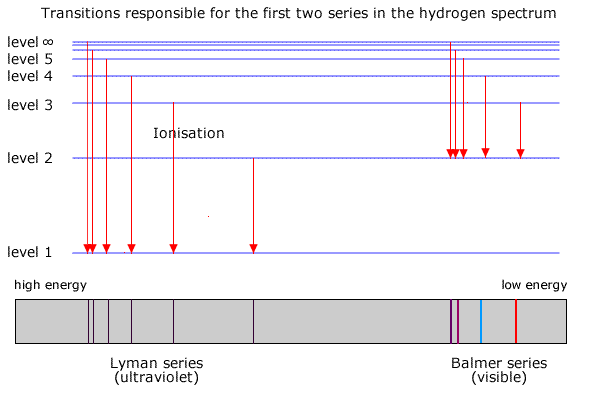
The value 109677 cm -1 is known as Rydberg constant for hydrogen. Electron transition to n 1 are responsible for lines in the UV region. Outline the model of electron configuration deduced from the hydrogen line emission spectrum Bohrs model.
Which is the electron configuration of a chromium atom in the ground stateA. I and III only. Describe the emission spectrum of hydrogen.
II and III only. Distinguish between a continuous spectrum and a line spectrum. Electron transition to n 1 are responsible for lines in the UV region.
Where n 1 1234. The value 109677 cm-1 is known as Rydberg constant for hydrogen. 233 Explain how the lines in the emission spectrum of hydrogen are related to electron energy levels.
Explain how the lines in the emission spectrum of hydrogen are related to. You need to understand convergence production of U. To learn more about hydrogen emission spectrum download BYJUS The Learning App.
Outline how this spectrum is related to the energy levels in the hydrogen atom. The line emission spectrum of hydrogen provides evidence for the existence of electrons in discrete energy levels which converge at higher energies. Outline the model of electron configuration deduced from the hydrogen line emission spectrum.
The smallest arrow of X represents a violet line in the emission spectrum. Describe the emission spectrum of hydrogen. Each line is a specific energy value.
Its spectra consists of large number of lines of different series and frequencies. The various series are named for the atomic energy level they end on n 1. Hydrogen Emission Spectra Aim.
N 3 n 2 C. IB Chemistry Topic 8 Acids and bases HLSL. I II and III Total 1 mark.
Emission or absorption processes in hydrogen give rise to series which are sequences of lines corresponding to atomic transitions each ending or beginning with the same atomic state in hydrogen. The arrows of W represent emission in the UV region. Emission spectrum is of two types Continuous and Line Spectrum When an electric discharge is passed through Hydrogen gas.
An electron is promoted to a higher energy level. ν wave number of electromagnetic radiation. The line emission visible spectrum of hydrogen.
IB Chemistry Atomic Structure SLHL Title. Hydrogen Emission Spectroscopy Visible region Balmer Series Line Emission Spectra for Hydrogen Hydrogen discharge tube Excited state Hydrogen Emission Spectroscopy 5 4 3 n 5-2 n 3-2 n 4-2 2 λ 434nm Visible region Balmer Series n n 2 Ground state 1 f cλ 3 x 108434 x 10-9 690 x 1014 Hz λ 486nm λ 656nm f cλ 3 x 108656 x 10-9. Lines are produced when electrons move from higher to lower energy levels.
Test your knowledge on Emission spectrum. The arrows represent the transition of electrons to different energy levels when heat is supplied. N 2 n 1 B.
The general formula for the hydrogen emission spectrum is given by. The spectrum of hydrogen is particularly important in astronomy because most of the Universe is made of hydrogen. Describe the emission spectrum of hydrogen.
Describe how the relative atomic mass of a sample of calcium could be determined from its mass spectrum. Calculate non-integer relative atomic masses and abundance of isotopes from given data. Explain how a line in the visible emission spectrum of hydrogen arises.
N 2 n 1 1. Describe and explain the differences between a line spectrum and a continuous spectrum. There is a lot more to the hydrogen spectrum than the three lines you can see with the naked eye.
This suggests that electrons can only possess a limited choice of allowed energies. Light is emitted when the electron falls from one orbital to another of lower energy and the frequency of the light emitted corresponds exactly to the energy difference - symbolically ΔE hf as used in IBbook your Databook and Physicsbook or ΔE hν as in Chembook pg. In the emission spectrum of hydrogen which electronic transition would produce a line in the visible region of the electromagnetic spectrum.
178 but not ΔE hυ as in IBs answer. The Hydrogen emission series. Hydrogen has one of the simplest emission spectra as there is only one electron per atom that can absorb and re-emit energy.
Outline how this spectrum is related to the energy levels in the hydrogen atom. Which is the electron configuration of a chromium atom in the ground state. I and III only C.
N2 n1 1. Up to 24 cash back Describe how the mass spectrometer may be used to determine relative atomic mass using the 12C scale. The general formula for the hydrogen emission spectrum is given by.
Using Balmer-Rydberg equation to solve for photon energy for n3 to 2 transition. The emission spectrum of hydrogenSome of the most common and readily observable series have been named as shown in this image where n 1 is the ground state and n 2 are excited states. Which statements are correct for the emission spectrum of the hydrogen atom.
These packets of energy are called quanta plural quantum What you should notice about this spectrum is that the lines get closer together towards the blue end of the spectrum. Total 3 marks 4. I and II only B.
Emission spectrum and atomic spectra. Solving for wavelength of a line in UV region of hydrogen emission spectrum. At this new higher energy level the electron is unstable and falls to a lower energy level which produces a photon of light in the form of the line in the emission spectrum.
Extending hydrogens emission spectrum into the UV and IR. ν wavenumber of the electromagnetic radiation. The lines converge at lower energies.
Explain how the lines in the emission spectra of hydrogen are related to the energy levels of the electrons. The series limit where n 2 is infinite and n 1 1 corresponds to the ionization energy of hydrogen. The arrows of Y represent emission of electromagnetic waves with higher energy than those represented by X and W.
N 2 n 3. Draw the emission spectrum and then on hydrogen. ν 109677 1 n 1 2 1 n 2 2 Where n1 1234.
Atomic Theory 1 33 The Hydrogen Spectrum

Ib Chemistry Atomic Theory Wikibooks Open Books For An Open World

Ib Chemistry Standard Level Notes Electronic Arrangement

Hydrogen Emission Spectrum Ib Chemistry Revision Course Youtube

Ib Chemistry Atomic Theory Wikibooks Open Books For An Open World

2 2 Hydrogen Emission Spectrum Sl Youtube

Absorption And Emission Spectra Ib Physics Youtube

Ib Chemistry Syllabus And Notes

2 2 Hydrogen Emission Spectrum Sl Youtube

Ib Chemistry Standard Level Notes Electronic Arrangement

Emission Absorption Spectrum 7 1 3 Ib Dp Physics Sl Revision Notes 2016 Save My Exams

Ib Chemistry Standard Level Notes Electronic Arrangement

Ib Chemistry Syllabus And Notes

2 2 The Line Spectrum Of Hydrogen Sl Ib Chemistry Youtube
How Does The Line Emission Spectrum Of Hydrogen Provide Evidence For The Existence Of Electrons In Discrete Energy Levels Which Converge At Higher Energies Quora
Atomic Theory 1 33 The Hydrogen Spectrum

Comments
Post a Comment